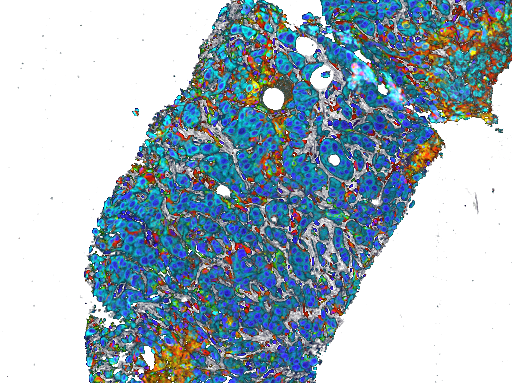
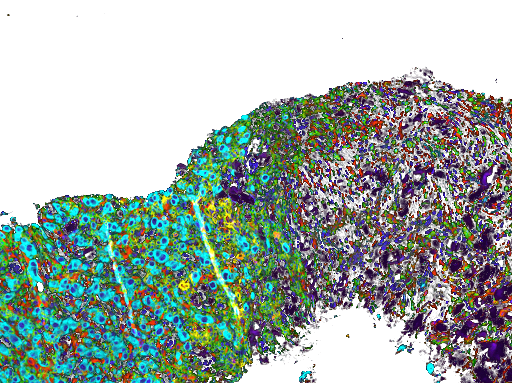
MEM-288 Tumor Biopsies
T-Cell Infiltrating Lymphocytes
Memgen is clinically testing its lead viral immunotherapy MEM-288 for advanced non-small cell lung cancer (NSCLC). MEM-288 combines our proprietary CD40 ligand with interferon beta (IFNβ) to provide the optimum capability to generate a robust, whole-body, tumor-targeted T cell immune response. The first stage of our phase 1 clinical trial recently completed treatment of 14 advanced NSCLC patients.
Safety
Only mild and transient treatment-related side effects such as injection site reactions and flu-like symptoms
Clinical Benefit
MEM-288 driven tumor shrinkage and disease control in 50% of advanced NSCL patients
Immune Activation
Induced anti-tumor immunity, including tumor specific T cell and dendritic cells, in both the tumor microenvironment and systemically throughout the body
Management Team and Board of Directors
We have a track record of success in the biotechnology, pharmaceutical, and finance sectors.

Director
Extensive career bridging scientific and financial expertise in life sciences. Founder and Vice Chairman of HealthCare Royalty Partners (“HCR”). MBA from Harvard Business School, an MD from SUNY Upstate College of Medicine, and a BA from Yale University.

Director
Extensive research and industry experience in cancer immunotherapy drug discovery and development. Inventor on multiple patented technologies, including Memgen’s viral cancer immunotherapies. PhD in Biomedical Sciences from the University of California San Diego.

Chairman of the Board
Dr. Coates is the founder and lead investor in Memgen. MBA and PhD in Finance, Accounting, and Economics from the University of Chicago Graduate School of Business.

Chief Financial Officer of Tracon Pharmaceuticals 2014-18 where she oversaw Tracon’s $41 million IPO and subsequent financings totaling over $100 million. Previous experience includes senior accounting positions at NuVasive and Orexigen Therapeutics and various CFO/accounting consulting roles.

Senior Vice President, Corporate & Business Development at Travere Therapeutics. Prior to Travere, Chief Business Officer at Tracon Pharmaceuticals. Before joining Tracon held senior corporate development roles at Bird Rock Bio and Anadys Pharmaceuticals (acquired by Roche).

Former Chief Investment Officer, Chief Financial Officer, and Chief Risk Officer with Assurant, Inc. a NYSE-traded insurance company, over twenty-three years. MBA from the University of Chicago Graduate School of Business and a BS in Economics from the University of Pennsylvania Wharton School.

Deeply experienced pharmaceutical senior executive. Former Global Head of Corporate Development at Abbott and AbbVie, responsible for multiple major acquisitions, including Pharmacyclics ($21 billion) and Knoll/BASF ($6.9 billion).

Founding member of Wiltshire, Whitley, Richardson & English, PA, a full-service accounting firm offering a comprehensive range of business and personal accounting services. He is a member of the American Institute of Certified Public Accountants (AICPA) and the Florida Institute of Certified Public Accountants (FICPA).
Scientific Advisory Board
Our board provides the expertise and guidance necessary to develop our cancer immunotherapies.
Our Science
Memgen’s
CD40 Ligand
A patented, safe and effective biological.
Memgen is applying its expertise in immune-regulatory systems to develop a range of immunotherapies, including viral immunotherapies, cell-based immunotherapies (TILs), and infectious disease vaccine adjuvants. Our immunotherapies are designed with multimodal mechanisms of action capable of working as single agents or in combination with checkpoint inhibitors and other therapies to maximize therapeutic benefit with a desirable safety profile.
Memgen’s immunotherapies leverage the power of our proprietary clinically evaluated CD40 ligand in combination with other co-stimulatory genes. MEM-288, our lead product candidate, combines our CD40 ligand with interferon beta (IFNβ). This combination has been engineered by Memgen and Moffitt Cancer Center to provide the optimum capability to generate a robust, whole-body, tumor-targeted T cell immune response.
The combination of a highly active viral immunotherapy with two potent transgenes turns Memgen's MEM-288 into a “triple threat” attacking cancers with three discrete mechanisms.
Partnering
Memgen is actively seeking strategic partnership and business opportunities with pharmaceutical and biotechnology companies for expanded research and clinical development.
Contact Us
Memgen's Headquarters
12 Greenway Plaza
Suite 1100
Houston, TX 77046
For company inquiries and partnering please contact:
Dr. Greg Brown, Memgen’s CEO
gbrown@memgenbio.com